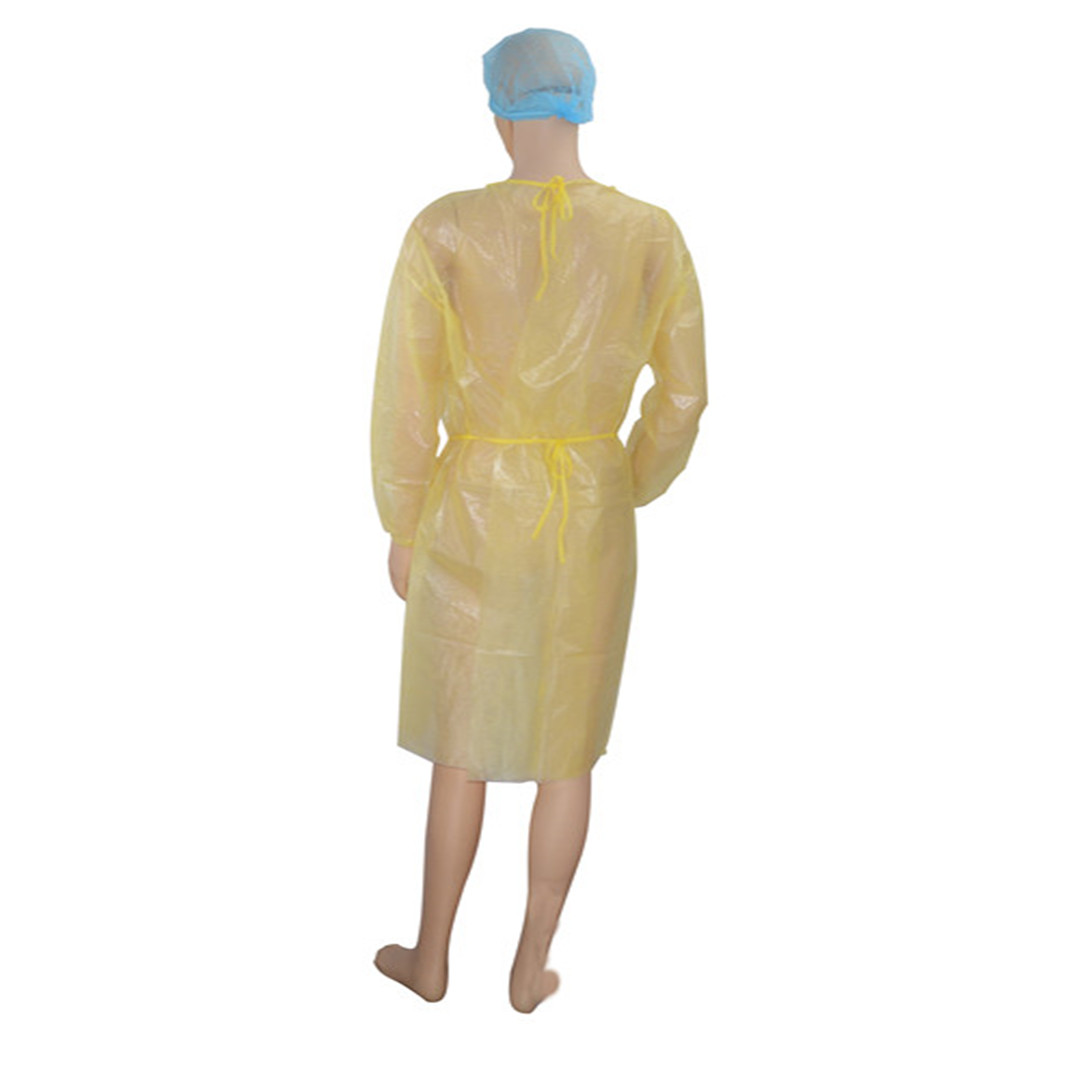
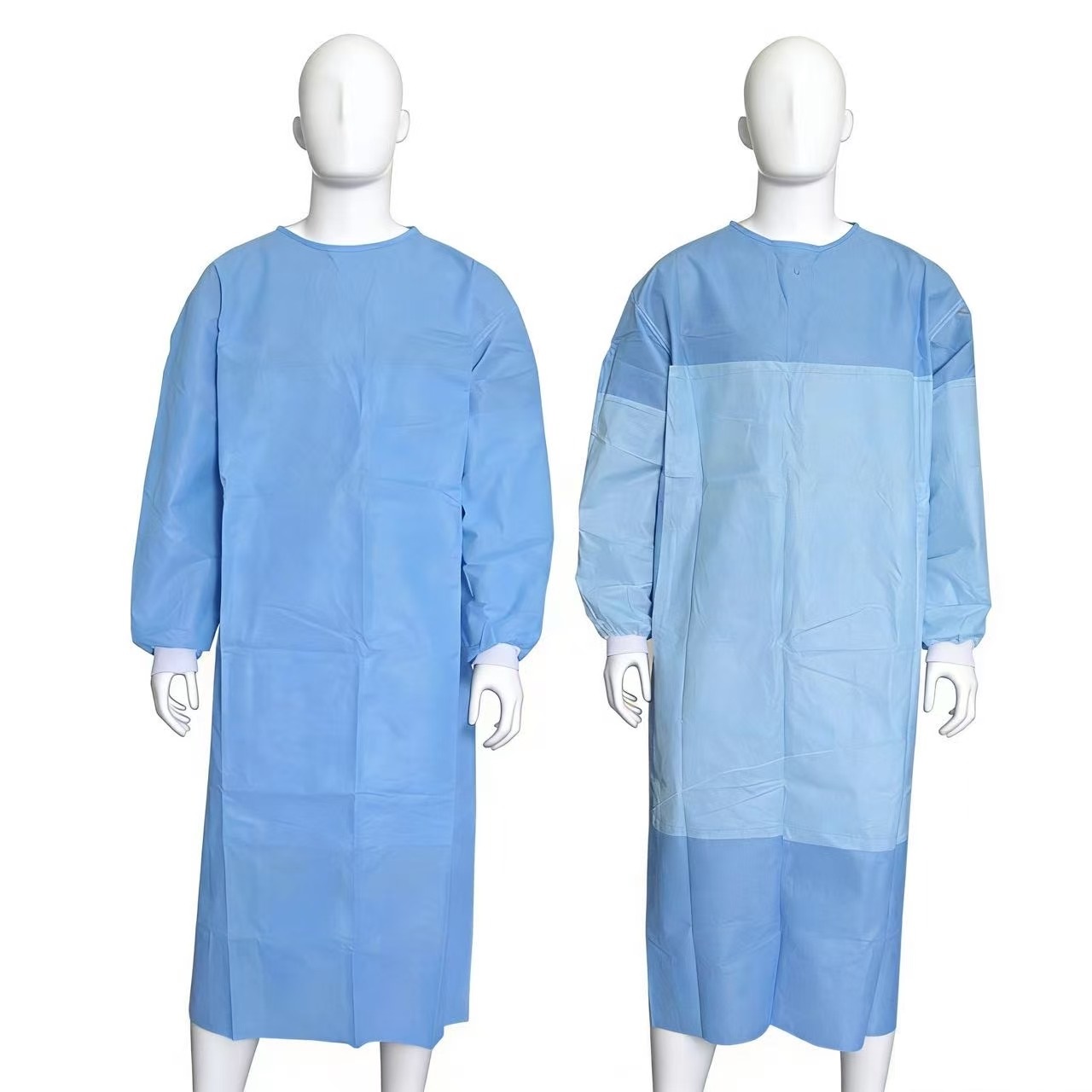
Size: S M L XL
Material: PP Non woven laminated PE film
Applied for medical and personal protective
Brand Name: OEM/ODM
Certification: EN14126, EN13795, FDA
Shipping Terms: EXW, FOB, FCA, CIF, etc
Minimum Order Quantity: Negotiable
Price: Negotiable
Packaging Details: EO Sterile, 1 pcs / peel pouch, 50 pcs / carton
Delivery Time: 30 days, depending on your order qty
Payment Terms: T/T, L/C, Western Union
Request A Quote